Pfizer's Covid Vaccine and Allergies: How Concerned Should You Be?
NY Times
DECEMBER 11, 2020
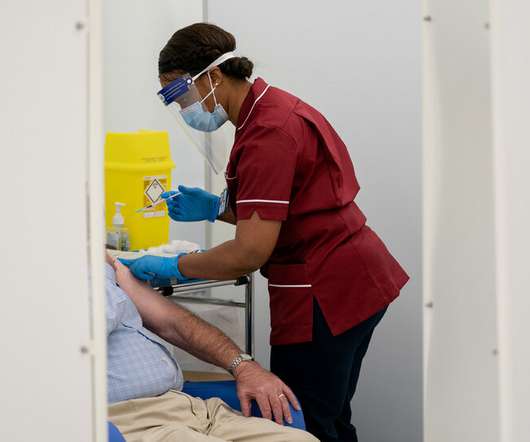
British health officials recommended that people with severe allergy reactions not be given the vaccine. Such reactions to vaccines are rare, even in people who have allergies to food or bee stings.


























Let's personalize your content