Dave Ross of Seqirus talks 'milestone' influenza vax vote
PharmaVoice
JULY 5, 2022
In a unanimous decision, a CDC committee calls for a preferential flu vaccine recommendation for people aged 65 and older for the first time.

PharmaVoice
JULY 5, 2022
In a unanimous decision, a CDC committee calls for a preferential flu vaccine recommendation for people aged 65 and older for the first time.

European Pharmaceutical Review
JULY 4, 2022
Ensuring potential microbial contaminants are not transferred into cleanroom environments is a key aspect of risk mitigation strategies. In a review, pharmaceutical microbiologist and contamination control expert Tim Sandle, reviewed several methods of material transfer, listing the decontamination methods based on efficacy and commenting on their weaknesses. .
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
JULY 6, 2022
Talks of a bear market in the biotech sector do not seem to pass, with news of companies falling into administration and layoffs becoming a recurrence. Just last week, trading for 4D Pharma , a British biotech, was suspended on the London Stock Exchange’s Alternative Investment Market , and the company will be delisted from NASDAQ on July 7. Elsewhere, San Diego, California-based Heron Therapeutics announced restructuring and layoffs for 34% of its workforce.

pharmaphorum
JULY 8, 2022
The pharmaceutical industry is increasingly relying on artificial intelligence to power its drug discovery and development efforts, and its spend in this area has created a multibillion-dollar market for AI technologies. That’s the conclusion of a GlobalData report , which notes that AI is being used to enhance computer-aided drug design (CADD) in a bid to reduce the time and costs involved in getting a new drug to market.

Speaker: Chris Antypas and Josh Halladay
Access to limited distribution drugs and payer contracts are key to pharmacy expansion. But how do you prepare your operations to take the next step? Meaningful data: Collect and share clinical data regarding outcomes, utilization, and more Reporting: Limited distribution models require efficient tracking and reporting systems Workflows: Align workflows with specific pharma and payer contractual requirements For in-depth, expert insights on pharmacy expansion, watch this webinar from Inovalon.

PharmaVoice
JULY 7, 2022
Precision BioSciences’ CEO discusses the company’s potentially groundbreaking cancer treatment.
European Pharmaceutical Review
JULY 8, 2022
As established facilities age, various risk considerations emerge. While some may continue to function effectively without any additional considerations, many will require additional checks and assessment. In an article published in the Journal of Validation Technology (JVT), pharmaceutical microbiologist and contamination control expert Tim Sandle identified ten categories of risk presented by aging.
Pharmacist Digest brings together the best content for pharmacists from the widest variety of industry thought leaders.

pharmaphorum
JULY 5, 2022
AstraZeneca has signed a deal to buy US biotech TeneoTwo in a deal worth up to $1.27 billion that will boost its position therapies for haematological cancers. The big pharma is paying $100 million upfront for the company, and offering up to $805 million in milestone payments if TeneoTwo’s drug candidates meet development objectives, plus up to $360 million if they hit sales targets.

PharmaVoice
JULY 5, 2022
AstraZeneca and Amgen are emphasizing diversity and authenticity in campaigns for lupus and severe asthma therapies.

European Pharmaceutical Review
JULY 5, 2022
After more than 20 years of environmental action, Pfizer has committed to further reduce its greenhouse gas (GHG) emissions and aims to achieve the voluntary Net-Zero Standard by 2040 – a full 10 years ahead of the standard’s proposed timeline. To achieve these goals, Pfizer aims to decrease its GHG emissions by 95 percent and its value chain emissions by 90 percent from 2019 levels by 2040.
Pharmaceutical Technology
JULY 4, 2022
Brii Biosciences (Brii Bio) has exercised an option for the acquisition of exclusive development and marketing rights for Vir Biotechnology’s investigational antibody, VIR-3434, for Hepatitis B in Greater China, under a partnership agreement. Following the option exercise, Vir will receive the option exercise fee, regulatory and commercial milestone payments as well as tiered royalties on net product sales from Brii Bio.

Advertisement
Are you still using workarounds to manage your daily operations? To achieve peak performance, it's time to explore other options for specialty and infusion pharmacy software. Streamline pharmacy operations and improve clinical performance with automated processing, real-time data exchange, and electronic decision support. Download this helpful infographic to: Drive efficiency and patient adherence from referral receipt to delivery and ongoing care – all with our Pharmacy Cloud.

pharmaphorum
JULY 4, 2022
GSK’s recently-unveiled plan to develop a bioscience cluster close to its main R&D site in Stevenage, UK, has moved closer to fruition, now that the first partners have joined the initiative. The asset management arm of Swiss back UBS has formed a joint venture with property developer Reef Group that will develop the 33-acre campus in Stevenage, Hertfordshire.

PharmaVoice
JULY 7, 2022
Marc Kikuchi weighs in on the company’s plans to pump up its pipeline and sales in the coming years.

PharmaTech
JULY 2, 2022
This article is focused on introducing a control chart technique using relative standard deviation (RSD) statistics (i.e., RSD chart); in other words, a coefficient of variation chart for continued process verification.
Pharmaceutical Technology
JULY 7, 2022
Brii Biosciences and TSB Therapeutics have commercially launched a long-acting neutralising antibody therapy combination, amubarvimab/romlusevimab, for Covid-19 in China. The two non-competing SARS-CoV-2 monoclonal neutralising antibodies, amubarvimab and romlusevimab are obtained from convalesced Covid-19 patients. The company developed these antibodies in partnership with Tsinghua University and the 3rd People’s Hospital of Shenzhen.

pharmaphorum
JULY 4, 2022
Today’s rare disease landscape comprises of around 7,000 different diseases. With a broad and diverse range of symptoms and severities impacting patients around the world, connecting these individuals with the information and expertise needed to achieve the correct diagnosis can be extremely lengthy and pose a significant barrier to treatment. “As the severity, progress, and treatment potential of rare diseases varies from patient to patient, each condition is going to need to be addressed diffe

PharmaVoice
JULY 6, 2022
From M&Ms to biotech, the chief financial officer's sweet tooth for taking risks is paying off.

European Pharmaceutical Review
JULY 7, 2022
Inadequate investigations and failure to follow standard operating procedures (SOPs) are some of the most common causes for regulatory warning letters in companies, according to Jeanne Moldenhauer, Vice President of Excellent Pharma Consulting. However, she notes that useful information for assessing risks in your facility and preparing for upcoming inspections can be gleaned from other’s warning letters and regulatory inspection observation reports, such as FDA 483.

Pharmaceutical Technology
JULY 6, 2022
The European Commission (EC) has granted approval for the expanded conditional marketing authorization (CMA) of Novavax’s Covid-19 vaccine, Nuvaxovid (NVX-CoV2373), in the European Union (EU) for adolescents of the age 12 to 17 years. A protein-based vaccine, NVX-CoV2373 is made from the genetic sequence of the SARS-CoV-2 virus’ first strain. The latest development comes after the Committee for Medicinal Products for Human Use of the European Medicines Agency granted a positive recommendation in

pharmaphorum
JULY 4, 2022
David Weingard, chief impact officer of T1D Moonshot and founder of Cecelia Health, tells us how T1D Moonshot aims to break silos and advance type 1 diabetes prevention and treatment options by utilising numerous partners’ expertise. In the US alone, 37.3 million Americans live with diabetes, with 1.6 million having type 1 diabetes (T1D). Still, a scarcity of T1D treatments exists.

PharmaVoice
JULY 6, 2022
Ratings agency Moody’s lays out the risk exposure for industry leaders.
Drug Patent Watch
JULY 5, 2022
Annual Drug Patent Expirations for MYCAPSSA Mycapssa is a drug marketed by Amryt and is included in one NDA. It is available from one supplier. There are eight patents protecting…. The post New patent for AMRYT drug MYCAPSSA appeared first on DrugPatentWatch - Make Better Decisions.
Pharmaceutical Technology
JULY 6, 2022
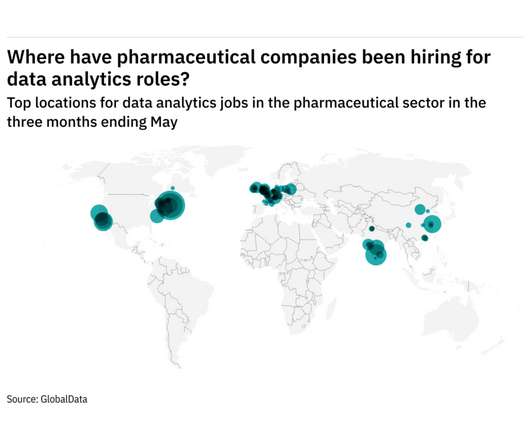
Europe was the fastest growing region for data analytics hiring among pharmaceutical industry companies in the three months ending May. The number of roles in Europe made up 11.8% of total data analytics jobs - up from 11.1% in the same quarter last year. That was followed by North America, which saw a -0.4 year-on-year percentage point change in data analytics roles.

pharmaphorum
JULY 5, 2022
Trial organisers face intense competition to find and recruit eligible patients for atopic dermatitis studies. With more than 500 dermatology clinical trials currently underway, it is often heard that there is a ‘’competition for participants’’. Yet evidence from research studies and a survey with 4,000+ atopic dermatitis patients conducted by Clariness shows that this ‘race to recruit’ is actually not the primary reason for the growing discontinuation rate of atopic dermatitis trials.

Pharma Times
JULY 7, 2022
The British Heart Foundation’s project will accelerate the search for better prevention and treatment of diabetes

Drug Patent Watch
JULY 5, 2022
Annual Drug Patent Expirations for SODIUM+NITRITE Sodium Nitrite is a drug marketed by Hope Pharms and is included in one NDA. It is available from one supplier. There is one…. The post New patent for Hope Pharms drug SODIUM NITRITE appeared first on DrugPatentWatch - Make Better Decisions.
Pharmaceutical Technology
JULY 8, 2022
Specialised Therapeutics’ oral drug, Nerlynx (neratinib) has obtained the Philippines Food and Drug Administration approval to lower the recurrence or mortality risk in early-stage HER2-positive (HER2+) breast cancer patients. The treatment is indicated as extended adjuvant treatment for adults with early-stage HER2-overexpressed/amplified breast cancer following adjuvant trastuzumab-based therapy.

pharmaphorum
JULY 5, 2022
I recently had the pleasure of attending the PING Conference 2022 , which had the tagline of ‘The Golden Age for Life Sciences Innovation’. In part, this was due to it being held at The Old Palace at Hatfield House, a location steeped in history and formerly the residence of Queen Elizabeth I, with whom ‘a golden age’ is often associated. However, it also reflected the optimistic tone of the event, organised by VWV , which aimed to present some rays of light for the future as we (hopefully) emer

European Pharmaceutical Review
JULY 5, 2022
Hereditary angioedema (HAE) is a genetic disorder, estimated to affect 1 in 50,000 people. It can lead to painful attacks of oedema (swelling) in various parts of the body, including the abdomen, face, feet, genitals, hands and throat. The main objective of the Phase III SPRING study ( NCT04070326 ) was to evaluate the safety and pharmacokinetics of Takhzyro ® (lanadelumab) – a fully human monoclonal antibody that specifically binds and decreases plasma kallikrein – in HAE patients aged two t

Pharma Times
JULY 5, 2022
Treatment abstract focuses on results from a multiple ascending dose study in healthy volunteers
Pharmaceutical Technology
JULY 7, 2022
Genetic mutations, both germline and acquired, are behind a large proportion of the most debilitating and sometimes life-threatening human diseases. But scientists have struggled to find effective treatments for many of these diseases since the dawn of modern medicine. For many decades, investigators have been working on innovative therapeutic modalities known as cell and gene therapies, which use modified versions of the body’s own cellular and genetic material to treat and potentially cure the

pharmaphorum
JULY 6, 2022
The FDA approves new cancer treatments in half the time of the EMA – but does faster mean better? And how can regulators balance timely access with robust safety? Cancer patients in Europe wait an average of almost eight months longer for access to breakthrough medications than their American counterparts. A new cross-sectional study has found that the FDA green lit 95% of the 89 new oncology therapies approved between 2010 and 2019 before the EMA, with the Europeans trailing the Americans by a

Drug Channels
JULY 6, 2022
The boom in copay accumulators and maximizers has radically shifted payers’ views on manufacturers’ copay support programs for specialty drugs. Below, I review a new survey of commercial plan sponsors and contrast the findings to a comparable survey from 2018. As you will see, plan sponsors now perceive manufacturers’ patient assistance programs primarily as a source of funding for their own plans.

European Pharmaceutical Review
JULY 7, 2022
Dr Eric Hughes joins Teva with experience in all phases of drug development at global pharmaceutical companies, most recently Boston-based Vertex Pharmaceuticals, which he joined last year. He will be based out of Teva’s US headquarters in Parsippany, New Jersey. . “Eric brings nearly 20 years of leadership roles with a proven track record in a variety of R&D functions for global pharmaceutical companies, and has a successful record of accomplishment fostering productive collaborations with
NY Times
JULY 3, 2022
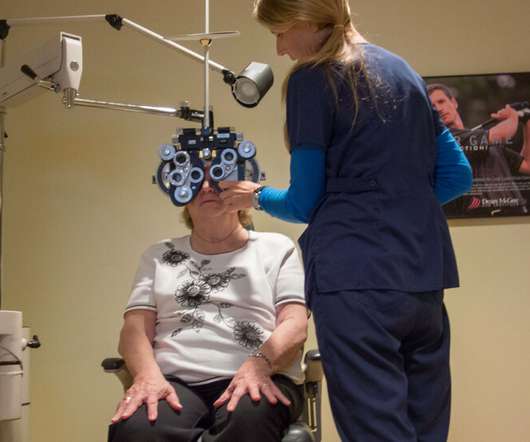
As experimental drugs prove ineffective against increasing dementia cases in the U.S., researchers argue that improving eyesight can have an effect.

pharmaphorum
JULY 5, 2022
Cell therapy specialist Mogrify has struck a deal with Japanese drugmaker Astellas to look at ways to deploy regenerative medicine to treat hearing loss caused by factors such as chronic exposure to loud noises. The two partners will take an in vivo approach to the problem of so-called sensorineural hearing loss (SNHL) looking at ways to replace sound-detecting hair cells in the inner ear (cochlea) that become damaged in this type of deafness.
Let's personalize your content