BioMarin resubmits haemophilia A gene therapy to the EMA
Pharma Times
JUNE 29, 2021
Company initially withdrew a marketing authorisation application for the gene therapy last year
Pharma Times
JUNE 29, 2021
Company initially withdrew a marketing authorisation application for the gene therapy last year
pharmaphorum
JULY 2, 2021
AstraZeneca has reported the first phase 2 results with a drug for heart failure with preserved ejection fraction (HFpEF), showing it worked as expected but wasn’t able to provide any clinical benefit to patients. . Daily doses of AZD4831 were able to reduce the activity of myeloperoxidase (MPO), an enzyme linked to tissue and blood vessel damage due to inflammation and scar tissues formation (fibrosis) in models of cardiovascular disease, by 69% after 30 days in the phase 2a SATELLITE stu
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

NY Times
JULY 1, 2021
The Pfizer and Moderna vaccines are safe and effective. Full approval from the F.D.A. will help stop the spread of Covid-19.
Outsourcing Pharma
JUNE 29, 2021
The trial solutions firm is working with Childrenâs Oncology Group to expand in-home research and care options for investigational immune-oncology therapy.

Speaker: Chris Antypas and Josh Halladay
Access to limited distribution drugs and payer contracts are key to pharmacy expansion. But how do you prepare your operations to take the next step? Meaningful data: Collect and share clinical data regarding outcomes, utilization, and more Reporting: Limited distribution models require efficient tracking and reporting systems Workflows: Align workflows with specific pharma and payer contractual requirements For in-depth, expert insights on pharmacy expansion, watch this webinar from Inovalon.
Pharma Times
JUNE 29, 2021
Mixing different vaccines produces a strong immune response against virus, according to researchers

pharmaphorum
JUNE 30, 2021
bluebird bio’s sickle cell disease (CD) gene therapy LentiGlobin is the latest recipient of an ‘innovation passport’ introduced in the UK earlier this year to speed up NHS access to promising new medicines. The designation means that LentiGlobin will be reviewed by the Medicines and Healthcare products Regulatory Agency (MHRA) via the new innovative licensing and access pathway (ILAP) introduced last December.
Pharmacist Digest brings together the best content for pharmacists from the widest variety of industry thought leaders.

Outsourcing Pharma
JUNE 28, 2021
The companyâs Ryze technology combines a clinical MDR and study automation platform, designed to accelerate and optimize design and execution of trials.

Pharma Times
JUNE 30, 2021
Innovation passport designation scheme aims to reduce the time to market for promising treatments
pharmaphorum
JUNE 30, 2021
Just a few months after starting clinical trials of its nasal spray vaccine for COVID-19, US biotech Altimmune is abandoning the project, saying that it generated weaker than expected immune responses in a phase 1 trial. . It’s a big disappointment for Altimmune, which said back in February that it was hoping to find a more convenient alternative to injected COVID-19 vaccines – and one which would be stable at room temperature, making distribution and delivery easier.

NY Times
JUNE 28, 2021
A new workshop explores the right of Indigenous people to govern the collection, ownership and use of their biological and cultural data.

Advertisement
Are you still using workarounds to manage your daily operations? To achieve peak performance, it's time to explore other options for specialty and infusion pharmacy software. Streamline pharmacy operations and improve clinical performance with automated processing, real-time data exchange, and electronic decision support. Download this helpful infographic to: Drive efficiency and patient adherence from referral receipt to delivery and ongoing care – all with our Pharmacy Cloud.
Drug Patent Watch
JULY 2, 2021
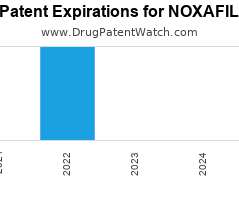
Annual Drug Patent Expirations for NOXAFIL Noxafil is a drug marketed by Merck Sharp Dohme and Schering and is included in three NDAs. It is available from three suppliers. There…. The post New patent for Merck Sharp drug NOXAFIL appeared first on DrugPatentWatch - Make Better Decisions.
Pharma Times
JULY 1, 2021
Checkpoint inhibitor approved in combination with chemotherapy in the first-line setting for these patients
pharmaphorum
JULY 1, 2021
Merck KGaA has joined forces with a unit of medtech firm B Braun – neuroloop – on a way to treat inflammatory diseases using neurostimulator devices. The German drugmaker will work with startup neuroloop on bioelectronics , put simply the harnessing of electrical stimulation to treat human disease, with a focus on chronic conditions like arthritis and inflammatory bowel disease. neuroloop – a spinout of Freiburg University in Germany formed in 2016 – has been working to date on using its device

NY Times
JUNE 29, 2021
New York’s sweeping lawsuit is the first opioid case in which a jury rather than a judge will decide the outcome.
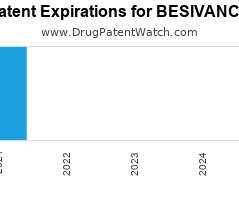
Drug Patent Watch
JUNE 28, 2021
Annual Drug Patent Expirations for BESIVANCE Besivance is a drug marketed by Bausch And Lomb and is included in one NDA. It is available from two suppliers. There are five…. The post New patent expiration for Bausch And drug BESIVANCE appeared first on DrugPatentWatch - Make Better Decisions.

Pharma Times
JUNE 29, 2021
Sanofi is currently collaborating with Translate Bio on a mRNA-based COVID-19 vaccine candidate

pharmaphorum
JUNE 30, 2021
Pharma has both an opportunity and a responsibility to do its part in tackling climate change – and the time to act is now, according to speakers at a recent event. Amanda Barrell reports. The pharma industry has clearly stated its commitment to a net zero future, but where are we on that journey, what else can be done, and could organisations achieve more if they worked together?
Outsourcing Pharma
JULY 1, 2021
On July 7, a group of seasoned industry leaders will offer insights at Rare/orphan diseases, special patient population, a focused, free online event.

Drug Patent Watch
JULY 2, 2021
Annual Drug Patent Expirations for INGREZZA Ingrezza is a drug marketed by Neurocrine and is included in one NDA. It is available from one supplier. There are fifteen patents protecting…. The post New patent for Neurocrine drug INGREZZA appeared first on DrugPatentWatch - Make Better Decisions.
Pharma Times
JUNE 30, 2021
Lenacapavir is designed to inhibit HIV-1 replication by interfering with a number of ‘essential’ steps in the viral lifecycle
pharmaphorum
JUNE 28, 2021
Exelixis has suffered a blow to its efforts to expand the use of Cabometyx into additional indications after reporting mixed results in a phase 3 trial in liver cancer. The COSMIC-312 study is comparing Cabometyx (cabozantinib) given alongside Roche’s cancer immunotherapy Tecentriq (atezolizumab) to Bayer’s Nexavar (sorafenib) as a first-line treatment for hepatocellular carcinoma (HCC), the most common form of liver cancer.

Drug Channels
JUNE 27, 2021
Happy 245th birthday, America! As you declare your vaccinated independence, celebrate with these Drug Channels fireworks: Amazon’s teeny step toward pharmacy disruption How patients view copay accumulators My $0.02 on generic and biosimilar trends Some hospitals seem to be gaming their 340B eligibility Plus, tips on effective hospital marketing. P.S.

Drug Patent Watch
JULY 2, 2021
Annual Drug Patent Expirations for PERSERIS+KIT Perseris Kit is a drug marketed by Indivior Inc and is included in one NDA. It is available from one supplier. There are seven…. The post New patent for Indivior Inc drug PERSERIS KIT appeared first on DrugPatentWatch - Make Better Decisions.
Pharma Times
JULY 2, 2021
Rylaze has been specifically developed for patients who have developed hypersensitivity to E.
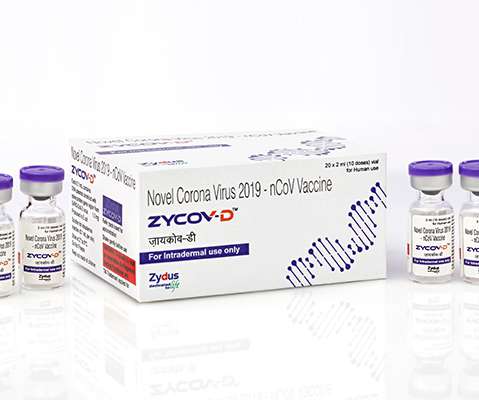
pharmaphorum
JULY 2, 2021
Zydus Cadila has filed for emergency use approval (EUA) in India of its plasmid DNA-based vaccine for COVID-19, which if given a green light could become the first shot of its type to be cleared for widespread use in humans. . Leaving aside the possible technological milestone, the ZyCOV-D vaccine has a few characteristics that could stand in the way of its rollout, including a need for three doses, whereas all India’s current vaccines only need two.

Outsourcing Pharma
JULY 1, 2021
Thanks in part to the recent completion of an $8m investment, 2020 On-site plans to expand its services, including the mobile support of vision research.
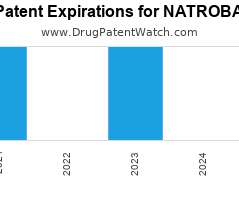
Drug Patent Watch
JULY 1, 2021
Annual Drug Patent Expirations for NATROBA Natroba is a drug marketed by Parapro Llc and is included in one NDA. It is available from two suppliers. There are two patents…. The post New patent expiration for Parapro Llc drug NATROBA appeared first on DrugPatentWatch - Make Better Decisions.

Pharma Times
JULY 1, 2021
Data also shows pharma companies worked with fewer HCPS and HCOs in 2020 compared to 2019

pharmaphorum
JUNE 29, 2021
Patient recruitment has begun in a National Institutes of Health (NIH) trial of two digital therapeutics (DTx) for people with opioid use disorder (OUD), developed by Pear Therapeutics and Chess Health. . The CTN-0100 study is test strategies to help keep people with OUD on drug treatment, improve the chances that those stabilised with drug treatments for OUD can come off medication without relapsing, as well as to find ways to predict the risk of relapse based on patient characteristics.

NY Times
JULY 2, 2021
Sean Doherty set up a private equity-like fund with other parents four years ago. Some investments in research have worked. Some haven’t.
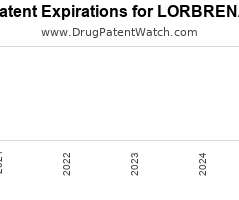
Drug Patent Watch
JULY 1, 2021
Annual Drug Patent Expirations for LORBRENA Lorbrena is a drug marketed by Pfizer and is included in one NDA. It is available from one supplier. There are two patents protecting…. The post New patent for Pfizer drug LORBRENA appeared first on DrugPatentWatch - Make Better Decisions.

Pharma Times
JUNE 29, 2021
The new Code has been updated to reflect changes in the pharma environment

pharmaphorum
JUNE 30, 2021
The University of Manchester has formed a joint venture with Morningside to carry out clinical trials of various digital health technologies developed by the investment group’s portfolio companies. . Health Innovation Manchester and its Academic Health Science Centre are also participating in the JV, which will focus on digital diagnostics and interventions that could play a role in prevention and early detection of disease and helping more patients to receive treatment outside the hospita
Outsourcing Pharma
JUNE 29, 2021
The organization, focused on building awareness and finding therapies for the rare disease, is boosting its annual research funding to more than $2.7m USD.
Drug Patent Watch
JUNE 30, 2021
Annual Drug Patent Expirations for LEXETTE Lexette is a drug marketed by Mayne Pharma and is included in one NDA. It is available from one supplier. There is one patent…. The post New patent for Mayne Pharma drug LEXETTE appeared first on DrugPatentWatch - Make Better Decisions.
Let's personalize your content