EU backs approval of Moderna’s COVID-19 vaccine in 12-17 year-olds
Pharma Times
JULY 27, 2021
Study in adolescents met it primary endpoint, successfully bridging immune responses to those observed in an efficacy study in adults
Pharma Times
JULY 27, 2021
Study in adolescents met it primary endpoint, successfully bridging immune responses to those observed in an efficacy study in adults
pharmaphorum
JULY 26, 2021
You’ve had your two COVID-19 jabs, but are you actually protected against infection? That’s a question that a fingerprick test launched today in the UK and Ireland could help to answer. The test – originally developed by US manufacturer Chembio Diagnostics – has been introduced into the UK and Irish markets by Guilford-based Luas Diagnostics and tests for the presence of SARS-CoV-2-targeting antibodies in the blood.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Eye on FDA
JULY 29, 2021
In the face of a crisis situation, it is a given that the clarity and thoroughness of the communications response is key to resolving the issue and mitigating any reputational damage. Perhaps no other decision by the Food and Drug Administration has garnered as much controversy, as the recent one to authorize the accelerated approval a new treatment for Alzheimer’s.

Outsourcing Pharma
JULY 27, 2021
A recent check-in with several biopharma and CRO companies, conducted by Life Science Strategy Group, indicates adjustment to pandemic-related challenges.

Speaker: Chris Antypas and Josh Halladay
Access to limited distribution drugs and payer contracts are key to pharmacy expansion. But how do you prepare your operations to take the next step? Meaningful data: Collect and share clinical data regarding outcomes, utilization, and more Reporting: Limited distribution models require efficient tracking and reporting systems Workflows: Align workflows with specific pharma and payer contractual requirements For in-depth, expert insights on pharmacy expansion, watch this webinar from Inovalon.
Pharma Times
JULY 28, 2021
Rate of rare blood clots with low platelets after the second dose is comparable to background rates observed in unvaccinated populations

pharmaphorum
JULY 26, 2021
A lack of racial diversity in clinical trials is a long-standing, well-documented problem that contributes to the stark health inequalities that have been brought into sharp focus by COVID-19. Tackling it, however, has not been easy, thanks in no small part to the reasons being as complex as they are multi-faceted. But, according to the Pharmaceutical Research and Manufacturers of America (PhRMA), the country’s biopharmaceutical companies are “committed to learning and leading” in a bid to “addr
Pharmacist Digest brings together the best content for pharmacists from the widest variety of industry thought leaders.
Outsourcing Pharma
JULY 27, 2021
The two pharma firms will partner to study the efficacy of Opdivo, paired with a SHP2 inhibitor, to treat non-small cell lung cancer with KRAS mutations.

Pharma Times
JULY 26, 2021
Combo therapy includes carflizomib administered alongside lenalidomide, dexamethasone and cyclophosphamide

pharmaphorum
JULY 27, 2021
Drug delivery specialist Aptar Group has agreed a deal to take a near two-thirds share in Voluntis, a French developer of digital therapeutics (DTx), with a view to taking full control of the company later this year. Illinois-headquartered Apar – which makes inhalers, syringe components, eye drop bottles and other delivery systems for pharmaceuticals, cosmetics and other sectors – is paying €8.70 per share in the initial deal, which will give it a 64.6% stake in the company.

Pharma in Brief
JULY 29, 2021
CADTH has convened a pan-Canadian Advisory Panel on a Framework for a Prescription Drug List (the Panel ). The Panel will provide recommendations on developing a potential pan-Canadian prescription drug list (or formulary). Stakeholder consultations are scheduled to take place in the fall and winter of 2021, leading to a final public report setting out the Panel’s non-binding recommendations in April 2022.

Advertisement
Are you still using workarounds to manage your daily operations? To achieve peak performance, it's time to explore other options for specialty and infusion pharmacy software. Streamline pharmacy operations and improve clinical performance with automated processing, real-time data exchange, and electronic decision support. Download this helpful infographic to: Drive efficiency and patient adherence from referral receipt to delivery and ongoing care – all with our Pharmacy Cloud.

Outsourcing Pharma
JULY 26, 2021
A new UK eLearning program is targeted at healthcare and academic professionals to support their learning on advanced therapy medicinal products (ATMPs).
Pharma Times
JULY 27, 2021
A traumatic injury includes any injury that requires admission to hospital at the time of injury, according to NICE

pharmaphorum
JULY 29, 2021
The use of specialty drugs in the U.S. has skyrocketed in recent years driven primarily by an increase in chronic condition diagnosis and the number of new medications on the market. Krishnanjan Alaparthi explores how tech-driven hub services can help manage the complexities of specialty pharma. It’s estimated specialty medications account for 75% of the approximately 7,000 prescription drugs currently in development, and by 2022, more than 60% of the 600 drugs expected to gain FDA approval will

NY Times
JULY 30, 2021
Emergent BioSolutions, which ruined 75 million vaccine doses at its Baltimore plant, disclosed records requests from Congress and federal and state law enforcement agencies.

Outsourcing Pharma
JULY 26, 2021
A clinical trial logistics expert discusses specialized concerns involved in transporting cell and gene products and other personalized medicine items.

Pharma Times
JULY 30, 2021
Although condition is extremely rare, it requires ‘swift diagnosis and urgent treatment’ says NICE
pharmaphorum
JULY 29, 2021
Two artificial intelligence (AI) algorithms designed to diagnose people with heart rhythm abnormalities have been approved by the FDA for use with Medtronic’s LINQ II cardiac monitor. The AccuRhythm algorithms can be used to improve the accuracy of detecting atrial fibrillation (AF) – an irregular or rapid rhythm in the upper chambers of the heart – and asystole, a long pause between heartbeats.

NY Times
JULY 29, 2021
Boosters may not be necessary yet, many experts say, and the pursuit of additional shots raises ethical questions.

Outsourcing Pharma
JULY 26, 2021
A clinical trial logistics expert discusses specialized concerns involved in transporting cell and gene products and other personalized medicine items.

Pharma Times
JULY 30, 2021
‘Genomics Nation’ report launched in a bid to showcase the thriving UK genomics sector
pharmaphorum
JULY 27, 2021
Flushed with the success of its COVID-19 vaccine, BioNTech has pressed the accelerator on the development of shots for other infectious diseases, and now plans to take malaria and tuberculosis candidates into the clinic next year. Human testing of the malaria shot should get underway by the end of 2022, according to the German biotech, which says it is working with the World Health Organization (WHO), European Commission and other organisations on the malaria project.

NY Times
JULY 27, 2021
If approved by a judge next month, the plan would resolve thousands of lawsuits and set in motion the release of $4.5 billion to help cover costs from the opioid epidemic.
Outsourcing Pharma
JULY 29, 2021
The BiovitalsHF, from Biofourmis, is intended to augment decision-making in clinical environments, and to supplement traditional pharmaceutical therapies.
Pharma Times
JULY 27, 2021
Organon will license the global development, manufacturing and commercial rights to investigational treatment ebopiprant

pharmaphorum
JULY 29, 2021
The UK has levied another big fine for anticompetitive activity in the pharma market in a fortnight, with Advanz Pharma and former owners on the hook for more than £100 million ($140 million) after increasing the price of a thyroid disease drug by 1,110% over an eight-year period. The Competition and Markets Authority (CMA) said the fine “sends a clear message” to the pharmaceutical industry that breaking the law will not be tolerated.

NY Times
JULY 28, 2021
The decision comes after state health officials were worried about whether doses of the Johnson & Johnson coronavirus vaccine would expire and go to waste.

Drug Channels
JULY 30, 2021
Today’s guest post comes from David Holladay, President of Access and Adherence at CoverMyMeds. David discusses how common medication access, adherence, and affordability barriers can lead patients to abandon important therapies. He suggests that technology can connect providers with actionable information at the point of care, thereby giving patients access to data that can lead them to make better decisions.

Pharma Times
JULY 29, 2021
CSU is the fifth disease for which Dupixent has positive Phase III data, according to Sanofi and Regeneron

pharmaphorum
JULY 29, 2021
Generic drugmaker Mylan has become the first company to secure FDA approval for a biosimilar product that is considered completely interchangeable with the reference product – namely Sanofi’s once-daily insulin Lantus. Mylan’s Semglee has been approved for over a year as a regular biosimilar to Lantus (insulin glargine), meaning that it could be used in place of Sanofi’s drug, but only if specifically prescribed for a patient.
NY Times
JULY 24, 2021
Muchos de los que recibieron la vacuna pueden necesitar refuerzos, dijeron los autores. Pero las autoridades sanitarias federales no recomiendan segundas dosis.
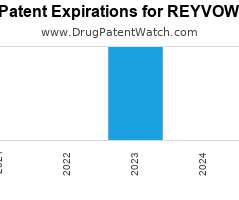
Drug Patent Watch
JULY 29, 2021
Annual Drug Patent Expirations for REYVOW Reyvow is a drug marketed by Eli Lilly And Co and is included in one NDA. It is available from one supplier. There are…. The post New patent for Eli Lilly drug REYVOW appeared first on DrugPatentWatch - Make Better Decisions.

Pharma Times
JULY 29, 2021
The company was co-founded in 2019 by Jeffrey Pollard and Luca Cassetta of the University of Edinburgh

pharmaphorum
JULY 29, 2021
AstraZeneca said this morning that it has made $1.2 billion in sales from its COVID-19 vaccine Vaxzevria in the first half of this year, but making it available at no profit had weighed on its profit margins. R&D expenses leaped 28% in the period, an increase that AZ said was “primarily” a result of its continued investment in the COVID-19 vaccine and other potential medicines to prevent and treat the coronavirus.

Pharma Marketing Network
JULY 28, 2021
Delivering a flawless customer journey is no longer beyond your reach. With precision analytics as an anchor behind data-driven decision-making, new advancements in data quality and access, plus rising cultural acceptance to innovation – we’re finally moving towards digital maturity. It’s time to enable more personalized touchpoint identification, channel selection and agile content needed to succeed in the hybrid future.
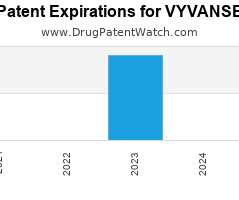
Drug Patent Watch
JULY 29, 2021
Annual Drug Patent Expirations for VYVANSE Vyvanse is a drug marketed by Takeda Pharms Usa and is included in two NDAs. It is available from one supplier. There are eighteen…. The post New patent for Takeda Pharms drug VYVANSE appeared first on DrugPatentWatch - Make Better Decisions.
Let's personalize your content