Drug-resistant epilepsy drug Ontozry backed by NICE
pharmaphorum
NOVEMBER 16, 2021
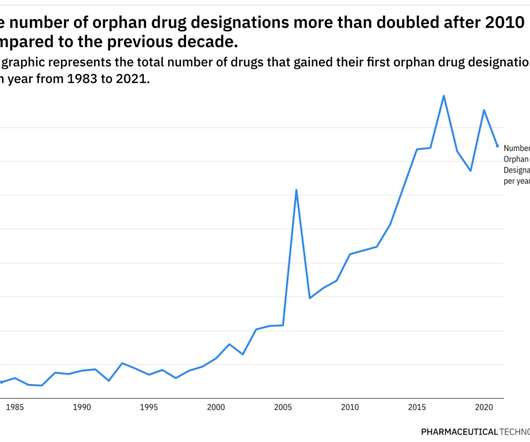
Focal onset seizure is a type of epilepsy in which seizures start in one side of the brain, acconting for around 60% of all epilepsy cases. “We look forward to the full technology appraisal guidance (TAG) which is due to be published in early December 2021,” he added.







































Let's personalize your content