Generative AI with Google Lens to detect counterfeits in pharma
Express Pharma
SEPTEMBER 8, 2023
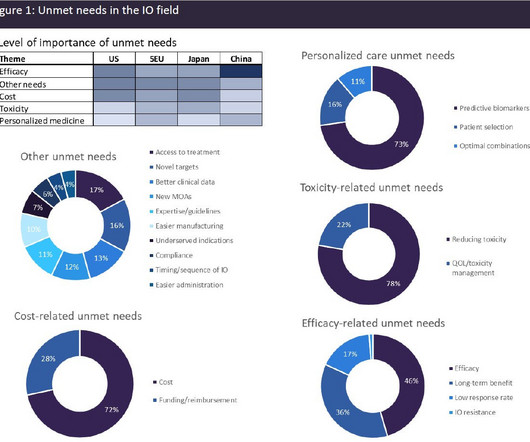
Combining generative AI and image recognition is probably the answer. I’d want to make a recommendation for pharma companies, government officials, and customers to combine the strength of AI with picture recognition to create a foolproof way to restrict the risks connected with counterfeit drugs. *In System workflow 1.


























Let's personalize your content