STAT+: CMS will use outcomes-based agreements in bid to help Medicaid pay for sickle cell gene therapies
STAT
JANUARY 30, 2024
In response to concerns over multimillion-dollar price tags for new gene therapies for sickle cell disease, the U.S. Two therapies recently approved by health regulators cost $2.2 million and $3.1 million, respectively, and have raised alarms over the ability of financially strapped state Medicaid programs to absorb the expense.







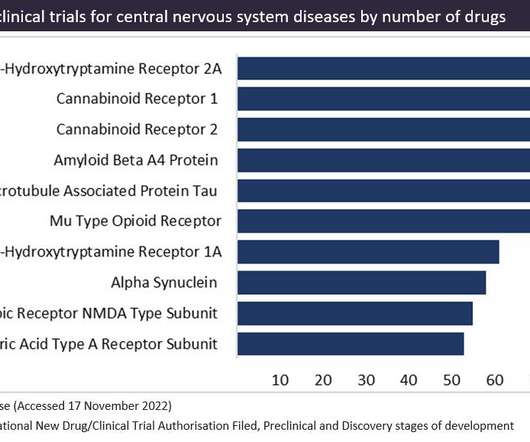














Let's personalize your content