Monitoring pharmaceuticals in the environment
European Pharmaceutical Review
APRIL 18, 2024
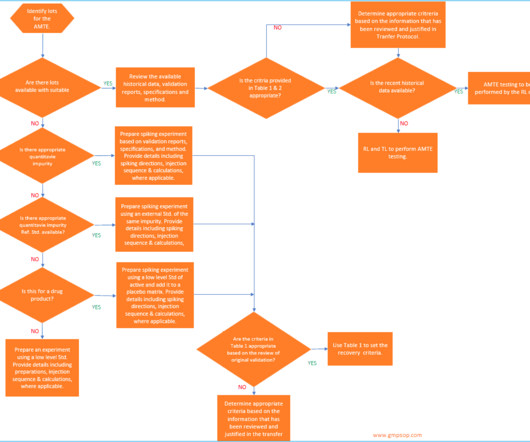
When measuring active pharmaceutical ingredient (API) concentrations in effluents, the limit of quantification (LoQ) of the chosen analytical method must be sensitive enough to measure anticipated effluent concentrations or risk-derived targets at the sampling point. Another important consideration is the sampling point.














Let's personalize your content