Nykode Therapeutics Expands its Clinical Collaboration and Supply Agreement with Roche to Evaluate VB10.16 for Advanced Cervical Cancer
PharmaShots
JUNE 1, 2023
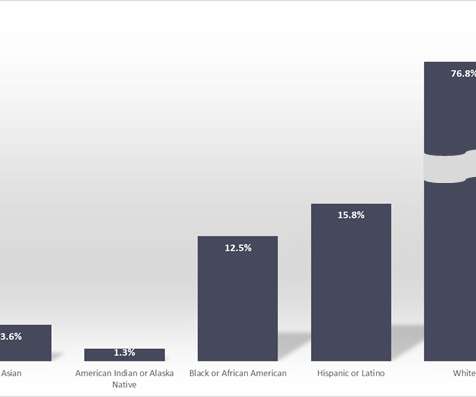
Shots: The companies expanded their collaboration to evaluate Nykode’s VB10.16, a wholly owned off-the-shelf therapeutic cancer vaccine candidate in the (VB-C-04) trial for patients with advanced cervical cancer in combination with Roche’s atezolizumab. at the time of analysis, and m-PFS (6.3mos.)





































Let's personalize your content