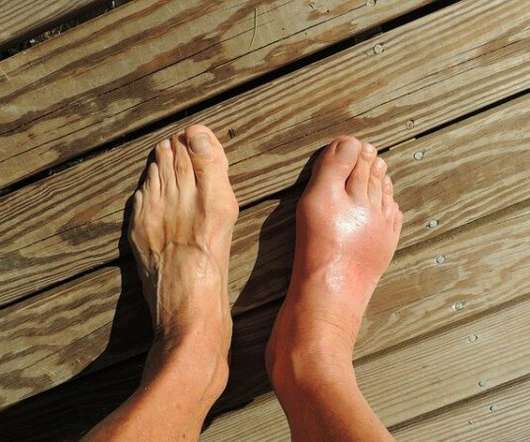
Gout market across 7MM set to reach $10.8 bn in 2032 fueled by novel ULTs and rising prevalence: GlobalData
Express Pharma
JANUARY 11, 2024
The sales growth of gout therapies across the seven major markets (7MM: the US, 5EU, and Japan) is set to increase at a compound annual growth rate (CAGR) of 8.8 These therapies are likely to adopted for patients with chronic gout or with hyperuricemia that is likely to lead to gout in the long-term. per cent from $4.6





























Let's personalize your content