Merck’s relapsing multiple sclerosis trial put on FDA partial clinical hold over liver injuries
Pharmafile
APRIL 14, 2023
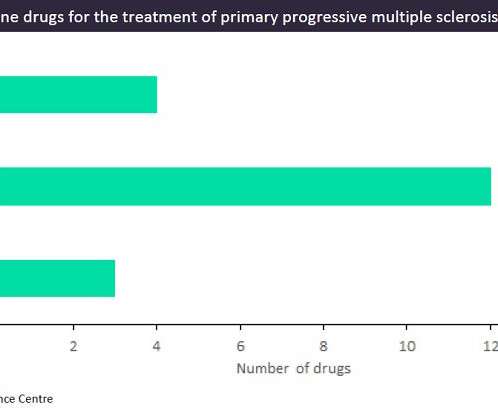
Merck has announced that its clinical phase 3 study EVOLUTION has been put on partial clinical hold by the FDA over liver injuries found in enrolled patients. EVOLUTION is a phase 3 clinical trial studying its investigational BTK inhibitor evobrutinib in relapsing multiple sclerosis (RMS). read more




































Let's personalize your content