Clinical Overview: Preventative Tools for Respiratory Syncytial Virus
Pharmacy Times
OCTOBER 23, 2023
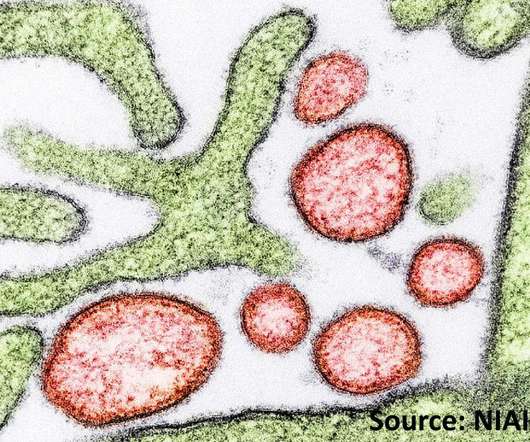
RSV can create a problematic infection among children 12 months of age or younger, older adults, and in patients with immunocompromised conditions.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

 clinical respiratory-syncytial-virus
clinical respiratory-syncytial-virus 
Pharmacy Times
OCTOBER 23, 2023
RSV can create a problematic infection among children 12 months of age or younger, older adults, and in patients with immunocompromised conditions.

World Pharma News
DECEMBER 12, 2023
NASDAQ: ICVX), a US-based clinical-stage biopharmaceutical company focused on developing differentiated, high-potential vaccines using an innovative, protein virus-like particle (VLP) platform. AstraZeneca has entered into a definitive agreement to acquire Icosavax, Inc.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

STAT
APRIL 9, 2024
A Pfizer vaccine showed the potential to protect adults ages 18 to 59 who are at increased risk of getting severely sick from respiratory syncytial virus in a late stage clinical trial, CNBC tells us. Landlords in places like greater Boston suddenly found they had vacant labs for the first time in a decade.
Pharmacy Times
SEPTEMBER 1, 2023
Pharmacists play a critical role in educating patients on RSV prevention, how to recognize early symptoms, and when to seek medical help.

PharmExec
DECEMBER 19, 2023
Pivotal trial findings show favorable clinical safety and efficacy data for mRNA-1345 in lowering the incidence of respiratory syncytial virus-associated lower respiratory tract disease.

World Pharma News
FEBRUARY 29, 2024
Pfizer Inc.

Pharmaceutical Technology
JANUARY 4, 2024
Palivizumab biosimilar is under clinical development by Mabxience Holding and currently in Phase I for Respiratory Syncytial Virus (RSV) Infections.

Express Pharma
AUGUST 21, 2023
ABRYSVO is unadjuvanted and composed of two preF proteins selected to optimise protection against RSV A and B strains and was observed to be safe and effective.

European Pharmaceutical Review
DECEMBER 22, 2022
It also has potential to develop vaccines for other respiratory diseases, such as flu and respiratory syncytial virus. This will include running a significant number of clinical trials in the UK. The Moderna Innovation and Technology Centre (MITC) is intended to provide access to a UK-made supply of COVID-19 jabs.
Pharmaceutical Technology
MARCH 21, 2023
Pre-clinical-stage biotechnology firm InvisiShield Technologies has partnered with Gladstone Institutes to develop intranasal preventatives against airborne viral infections including influenza, respiratory syncytial virus (RSV) and SARS-CoV-2.

Pharmaceutical Technology
JUNE 8, 2023
The European Commission (EC) has granted marketing authorisation for GSK’s respiratory syncytial virus (RSV) vaccine, Arexvy, for adults aged 60 years and above. The vaccine is indicated for active immunisation to prevent RSV-related lower respiratory tract disease (LRTD) in older adults.

Express Pharma
MAY 3, 2023
GSK announced that the US Food and Drug Administration (FDA) has approved Arexvy (respiratory syncytial virus vaccine, adjuvanted) for the prevention of lower respiratory tract disease (LRTD) caused by respiratory syncytial virus (RSV) in individuals 60 years of age and older. per cent (96.95% CI, 57.9–94.1,

pharmaphorum
DECEMBER 15, 2022
However, this has led to increased transmission of other respiratory diseases and, as we’re already seeing, there are now some very real concerns about a lesser-known virus – respiratory syncytial virus, or RSV. RSV is one of the most common respiratory viruses, causing coughs and colds during the winter months.
Pharmafile
APRIL 12, 2023
Biotechnology company Moderna has announced clinical updates detailing the expansion and advancement of its mRNA pipeline, which includes treatments for COVID-19, influenza and respiratory syncytial virus (RSV), amongst others.
Pharmaceutical Technology
APRIL 7, 2023
Enanta Pharmaceuticals has secured the US Food and Drug Administration’s (FDA) fast track designation for EDP-323 to treat respiratory syncytial virus (RSV). Consistent potency was also observed across a range of RSV clinical isolates in several cell types.
Pharmafile
DECEMBER 23, 2022
The aim of the partnership is to generate a ready supply of UK-produced mRNA vaccines for COVID-19 and other infectious diseases like flu and respiratory syncytial virus (RSV). read more.
Pharmafile
JANUARY 5, 2023
The FDA has accepted an application for nirsevimab as the first protective option to combat respiratory syncytial virus (RSV) disease for all infants. If approved, the drug would become the first broadly protection option for RSV.

PharmaShots
APRIL 10, 2023
Shots: The US FDA has granted FTD for Enanta’s EDP-323 (L-protein inhibitor) for the treatment of the respiratory syncytial virus. EDP-323 is being studied in a P-I study evaluating the safety, tolerability, and PK with expected results in Q2’23 The company plans to present new preclinical PK data at ECCMID 20323.

Express Pharma
JANUARY 29, 2024
GlobalData’s latest report, “mRNA Vaccines in Infectious Diseases-Market Overview”, reveals that several late-stage pipeline products are in development for various infectious diseases, such as influenza, respiratory syncytial virus (RSV), and cytomegalovirus, as well as for COVID-19.
European Pharmaceutical Review
AUGUST 22, 2023
Abrysvo is approved for pregnant individuals to prevent lower respiratory tract disease (LRTD) and severe LRTD caused by respiratory syncytial virus (RSV) in infants. The US Food and Drug Administration (FDA)-approved treatment, is the first RSV vaccine indicated for infants from birth to six months of age.
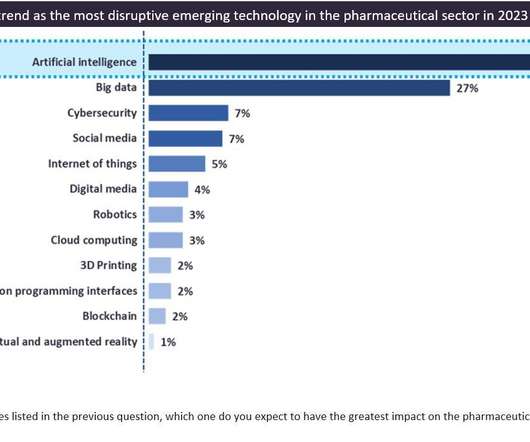
Pharmaceutical Technology
JANUARY 16, 2023
Recent cases in the pharmaceutical sector include Poolbeg Pharma finding more than one respiratory syncytial virus (RSV) vaccine candidate with AI by going through a vast amount of early stage clinical data to prioritise candidates and reposition them as novel treatments for RSV infection.

pharmaphorum
DECEMBER 22, 2022
It also said the alliance will see Moderna invest in R&D in the UK over the next 10 years, including running a number of clinical trials and providing grant funding to UK universities, while the manufacturing facility will create more than 150 highly skilled jobs. The first vaccines are due to be produced at the new facility in 2025.
pharmaphorum
JULY 12, 2022
Researchers in the US have started a clinical trial of a vaccine against Nipah virus, a serious infection caught from animals that has a fatality rate of between 40% and 70%, developed by mRNA specialist Moderna. “The need for a preventive Nipah virus vaccine is significant,” he added.

pharmaphorum
DECEMBER 1, 2021
The vaccine has just started clinical testing, but is designed to control the growth of C acnes on the skin and prevent it from damaging the cellular lining of pores, which can lead to scarring in severe acne cases. It will highlight vaccines for pneumococcal disease, meningitis, respiratory syncytial virus (RSV), influenza, and chlamydia.
Pharmaceutical Technology
FEBRUARY 23, 2023
This collaboration is expected to help expedite the clinical manufacturing of RVM-V001 and future mRNA-based vaccines that target infectious diseases such as Clostriodioides difficile infection (CDI) and Respiratory syncytial virus (RSV).

PharmaShots
JUNE 23, 2023
Gilead Receives EMA’s CHMP Positive Opinion of Trodelvy (sacituzumab govitecan) for Pre-Treated HR+/HER2- Metastatic Breast Cancer Date: June 23, 2023 | Tags: Gilead, Trodelvy, sacituzumab govitecan, HR+/HER2- Metastatic Breast Cancer, Regulatory, EMA, CHMP, Positive Opinion Sarepta Therapeutics’ Elevidys Receives the US FDA’s Accelerated (..)
pharmaphorum
JULY 21, 2022
GSK’s former head of vaccines R&D – Dr Emmanuel Hanon – is heading up a new biotech called Vicebio that will go up against his former employer with a vaccine against respiratory syncytial virus (RSV) infections. ” He said. ” The post Ex-GSK vaccine chief to lead new UK biotech Vicebio appeared first on.

pharmaphorum
JUNE 29, 2022
Already competing to bring the first vaccine against respiratory syncytial virus (RSV) to market, Pfizer and GSK are also fighting a patent infringement battle in the courts. com website.
Pharmaceutical Technology
NOVEMBER 21, 2022
This will aid in the production of new raw materials and possible clinical-grade assets to develop mRNA vaccines and therapies for infectious diseases, as well as other ailments with unmet needs. It commenced a first-in-human clinical trial of the vaccines in September this year. .

pharmaphorum
OCTOBER 12, 2022
“Continuing our strategic alliance with Merck is an important milestone as we continue to grow our mRNA platform with promising clinical programmes in multiple therapeutic areas.” The post Merck gets on board with Moderna cancer vaccine in $250m deal appeared first on.

Pharmaceutical Technology
JUNE 6, 2023
According to Globaldata, it is involved in 5 clinical trials, which are ongoing. The rNPV model is a more conservative valuation measure that accounts for the risk of a drug in clinical development failing to progress. Pharma Mar also offers kits used for diagnosis of influenza A and B and respiratory syncytial virus.
pharmaphorum
OCTOBER 13, 2022
GSK’s vaccine against respiratory syncytial virus (RSV) is one of the top prospects in its pipeline, but also one with considerable competition, so needs strong data to support the programme. Top of the list is an overall protective efficacy against RSV lower respiratory tract disease of 82.6%

pharmaphorum
NOVEMBER 30, 2021
Dormitzer also took the lead on Pfizer’s respiratory syncytial virus (RSV) vaccine candidates, which are in late-stage testing and a race to market with GSK own experimental shots, as well as a BioNTech-partnered influenza jab BNT161, which started clinical trials earlier this year.

pharmaphorum
JUNE 23, 2022
Enanta has a long heritage in the development of antiviral compounds, working with AbbVie on therapies for hepatitis C virus (HCV) infection including Viekira Pak (paritaprevir/ritonavir/ombitasvir/dasabuvir) and Mavyret/Maviret (glecaprevir/pibrentasvir).

pharmaphorum
MAY 9, 2022
Johnson & Johnson has halved the size of its collaboration with Bavarian Nordic on vaccine development, jettisoning its stake in shots targeting human papillomavirus (HPV) and hepatitis B virus (HBV). ” The partners’ HPV candidate had advanced into phase 1/2 clinical trials.

pharmaphorum
JANUARY 18, 2023
Moderna is looking to make a swift dash to the finish line with its respiratory syncytial virus (RSV) vaccine candidate, based on the same mRNA technology as its COVID-19 shots, with a filing in with the FDA in the first half of this year. Look out, Pfizer and GSK. for placebo.
pharmaphorum
MARCH 22, 2022
Bavarian Nordic has licensed rights to its respiratory syncytial virus (RSV) vaccine in China and certain other Asian markets to Shanghai’s Nuance Pharma in a $225 million deal. million tied to clinical, regulatory, and commercial milestones as well as royalties on sales if MVA-BN RSV is launched in those markets.

PharmaShots
APRIL 14, 2023
Ocugen Reports Preliminary Results from the P-I/II Trial of OCU400 for the Treatment of Retinitis Pigmentosa and Leber Congenital Amaurosis Date: Apr 14, 2023 | Tags: Ocugen, OCU400, Retinitis Pigmentosa, Leber Congenital Amaurosis, Clinical Trial, P-I/II Trial Candesant Biomedical Receives the US FDA Clearance of Brella SweatControl Patch for Primary (..)

pharmaphorum
JUNE 23, 2021
GlaxoSmithKline’s pitch to shareholders kicked off this afternoon with an optimistic view of its late-stage pipeline – including some big sales predictions for products like its respiratory syncytial virus (RSV) vaccine and new blood cancer drug Blenrep. billion takeover of Tesaro in 2019. £1
pharmaphorum
JUNE 11, 2021
“Based on the robust clinical evidence we have to-date, sutimlimab significantly inhibits haemolysis and has the potential to be an important new treatment for CAD,” said Karin Knobe, head of development, rare, and rare blood disorders at Sanofi.

Digital Pharmacist
AUGUST 31, 2023
Whether addressing acute pharyngitis, influenza, COVID-19, or other respiratory infections, the ability to swiftly identify causative agents empowers healthcare providers to make informed decisions that significantly impact patient outcomes. Navigating State Regulations for POCT State regulations influence rapid diagnostic testing protocols.

pharmaphorum
JANUARY 21, 2021
Capsule’s software is already used by more than 2,800 healthcare organisations around the world for device integration, vital signs monitoring and clinical surveillance services, including in intensive care units. That agreement comes a few weeks after Philips signed a $2.8 to develop a digital approach to personalised fertility treatment.

pharmaphorum
JUNE 29, 2021
Sanofi has just significantly increased its investment in mRNA vaccine development, earmarking €400 million ($475 million) a year in R&D funding to an effort that it hopes will deliver at least six clinical-stage candidates by 2025. .

pharmaphorum
JUNE 22, 2022
It will also serve as the focal point of a clinical trials hub for Moderna, and a collaboration with the UK health services on R&D. Construction of the facility is expected to start later this year, said the government, with the first mRNA vaccines due to be produced there in 2025.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?

Let's personalize your content