STAT+: Biotech launches with $82 million for Parkinson’s cell therapy
STAT
FEBRUARY 29, 2024
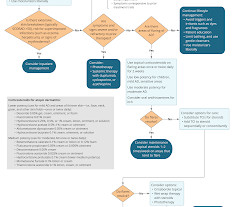
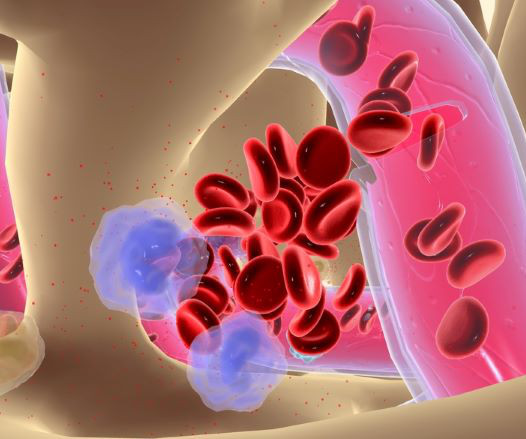
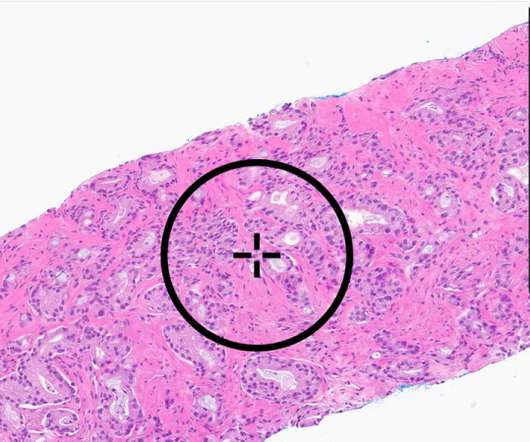
The dream of being able to pluck a cell therapy off of a freezer shelf and speedily give it to cancer patients is materializing in the cancer world. These highly versatile organisms are derived from skin or blood cells and can be transformed into any type of cell.



































Let's personalize your content