STAT+: FDA approves Medtronic high blood pressure device despite a negative advisory panel vote
STAT
NOVEMBER 17, 2023
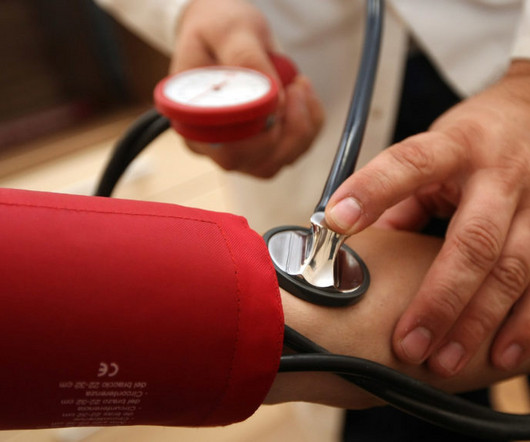
The FDA approved a similar product, part of a device class called renal denervation, from startup Recor Medical earlier this month. “We partnered closely with leading experts in our clinical community who could help us in our journey to get this technology to the people who need it most.

















































Let's personalize your content