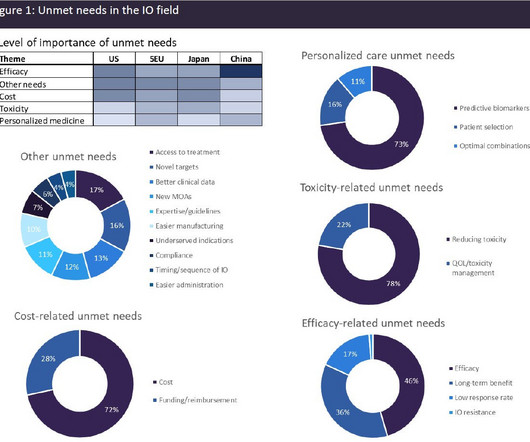
Roche nabs breakthrough tag for TIGIT cancer immunotherapy
pharmaphorum
JANUARY 5, 2021
Roche’s closely-watched combination of two checkpoint inhibitors – TIGIT-targeting tiragolumab and PD-L1 drug Tecentriq – has claimed breakthrough status from the FDA. The post Roche nabs breakthrough tag for TIGIT cancer immunotherapy appeared first on.










Let's personalize your content