Developing point-of-care CAR T manufacturing
European Pharmaceutical Review
DECEMBER 27, 2023
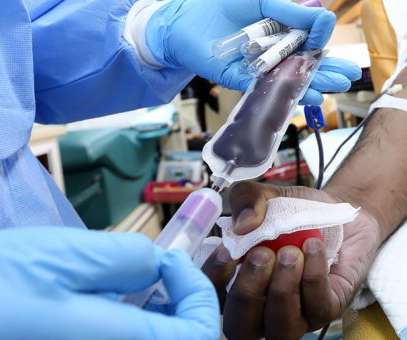
Chimeric antigen receptor (CAR) T cells are the first example of a “living drug”. 1 Following in vitro production from T cells collected from a patient’s leukapheresis procedure, the CAR T cells are infused back into the patient’s blood, where they proliferate and expand. This is variable and to some extent unpredictable.













Let's personalize your content