Genprex’s Reqorsa gene therapy picks up orphan drug designation from FDA for SCLC
Pharmaceutical Technology
AUGUST 11, 2023
The latest tag adds to three fast track designations for Reqorsa, with the company initiating a Phase I/II trial in Q4 2023.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

 tag tracking
tag tracking 
Pharmaceutical Technology
AUGUST 11, 2023
The latest tag adds to three fast track designations for Reqorsa, with the company initiating a Phase I/II trial in Q4 2023.

Pharmaceutical Technology
AUGUST 1, 2023
Vepdegestrant was awarded the Innovation Passport designation for treating advanced or metastatic breast cancer.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

PharmaShots
APRIL 14, 2023
Ocugen Reports Preliminary Results from the P-I/II Trial of OCU400 for the Treatment of Retinitis Pigmentosa and Leber Congenital Amaurosis Date: Apr 14, 2023 | Tags: Ocugen, OCU400, Retinitis Pigmentosa, Leber Congenital Amaurosis, Clinical Trial, P-I/II Trial Candesant Biomedical Receives the US FDA Clearance of Brella SweatControl Patch for Primary (..)

European Pharmaceutical Review
APRIL 19, 2023
Smart packaging has potential for the integration of electronic functionality, enabling compliance, material identification, condition monitoring and asset tracking of pharmaceutical products. Yet apart from radio-frequency identification (RFID) tags and QR codes, adoption at scale has proved challenging overall, the report stated.
pharmaphorum
DECEMBER 24, 2021
Biogen and Eisai head towards the end of the year with some much-needed good news in their Alzheimer’s programmes, as the FDA awards a fast-track designation to lecanemab, their follow-up to recently approved Aduhelm. The post Biogen, Eisai Alzheimer’s drug lecanemab fast tracked by FDA appeared first on.

Express Pharma
SEPTEMBER 8, 2023
Generative AI will be used to create unique invisible image tags for each product (like UID). This can be done by training the AI on a dataset of existing batch numbers, allowing it to generate new, unique invisible image tags on brand logo / brand name that cannot be replicated by counterfeiters 2. Key features and benefits a.

Pharmaceutical Technology
SEPTEMBER 30, 2022
Spark Therapeutics’ Luxturna, indicated for inherited retinal disease (IRD), was the first gene therapy to be approved, in 2017, with a price tag of $850,000 for each eye. Prior to bluebird's approvals, there were only two FDA-approved gene therapies for inherited conditions on the market.
RX Note
MAY 16, 2023
Introduction Google Analytics is a free platform offered by Google that helps you track and analyze website traffic. Also, Google Analytics can track your website's bounce rate, which is the percentage of visitors who leave your blog after viewing only one page. Upon account creation, you will receive a tracking ID.

The Digital Apothecary
NOVEMBER 12, 2018
Can a time tracking system save me time? Why I bought a time tracking service Recently I made the plunge and bought a time tracking tool. Now, I have used multiple platforms over the years, and if you read my blog a lot you probably notice I have used different visuals to keep track of what I am up to throughout the day.
Pharmaceutical Technology
JUNE 29, 2022
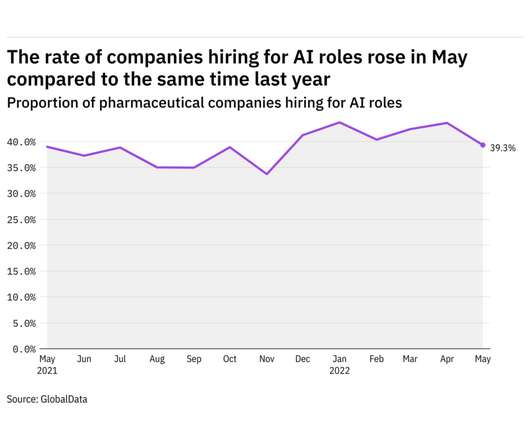
GlobalData's job analytics database tracks the daily hiring patterns of thousands of companies across the world, drawing in jobs as they're posted and tagging them with additional layers of data on everything from the seniority of each position to whether a job is linked to wider industry trends. in May 2022.

pharmaphorum
OCTOBER 18, 2022
Medtech giant Beckton Dickinson (BD) has signed a deal with France’s Biocorp to use the latter’s near-field communication (NFC) tags in injectable devices. The Injay tag can confirm a complete injection and transfer that information via an NFC reader to a smartphone or tablet for review by a healthcare professional.
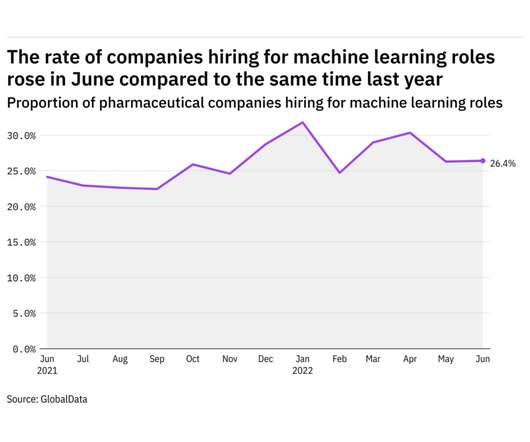
Pharmaceutical Technology
JULY 25, 2022
GlobalData's job analytics database tracks the daily hiring patterns of thousands of companies across the world, drawing in jobs as they're posted and tagging them with additional layers of data on everything from the seniority of each position to whether a job is linked to wider industry trends. in June 2022.
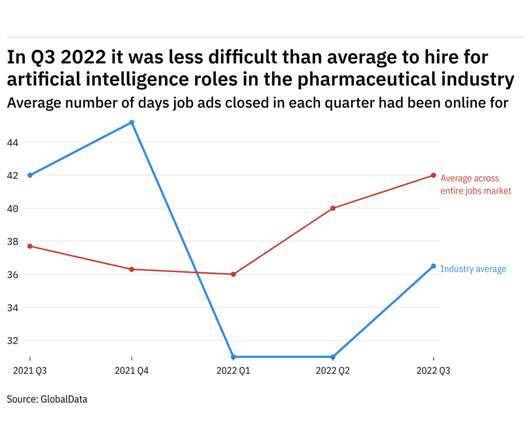
Pharmaceutical Technology
NOVEMBER 7, 2022
GlobalData's job analytics database tracks the daily hiring patterns of thousands of companies across the world, drawing in jobs as they're posted and tagging them with additional layers of data on everything from the seniority of each position to whether a job is linked to wider industry trends.

European Pharmaceutical Review
MAY 22, 2023
Smart contracts, which are essential for easing international trade and logistics operations, can enable the user to track fake returns to the producer and supplier using RFID tags. Blockchain offers the possibility of non-modifiable data uploading with traceability and low-cost data storage.
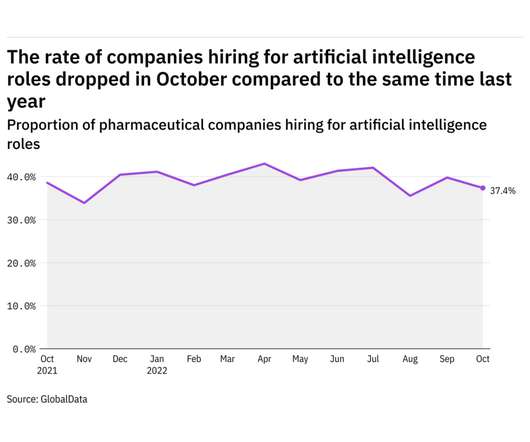
Pharmaceutical Technology
NOVEMBER 9, 2022
GlobalData's job analytics database tracks the daily hiring patterns of thousands of companies across the world, drawing in jobs as they're posted and tagging them with additional layers of data on everything from the seniority of each position to whether a job is linked to wider industry trends. in October 2022.

Viseven
MAY 24, 2023
There are countless possible applications of AI, including symptom tracking with respective warnings and education – these use cases were also covered by Reuters Pharma speakers.
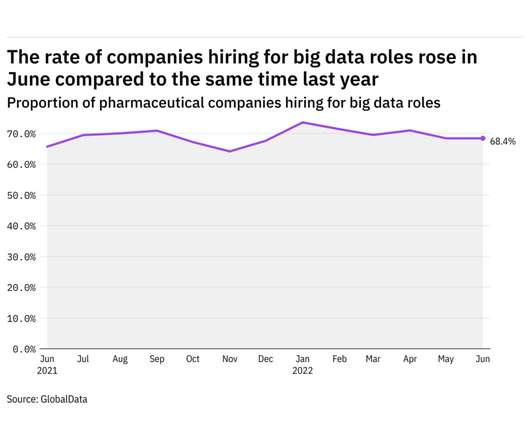
Pharmaceutical Technology
JULY 6, 2022
GlobalData's job analytics database tracks the daily hiring patterns of thousands of companies across the world, drawing in jobs as they're posted and tagging them with additional layers of data on everything from the seniority of each position to whether a job is linked to wider industry trends. in June 2022.
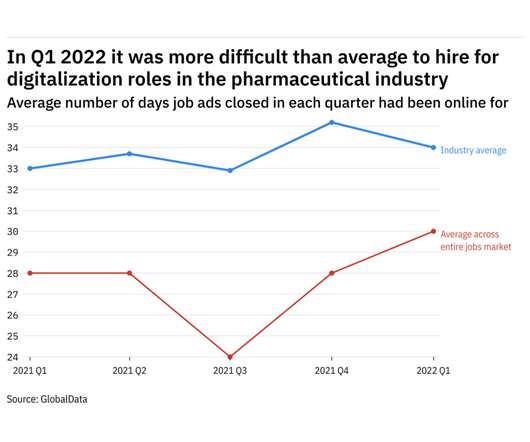
Pharmaceutical Technology
JULY 8, 2022
GlobalData's job analytics database tracks the daily hiring patterns of thousands of companies across the world, drawing in jobs as they're posted and tagging them with additional layers of data on everything from the seniority of each position to whether a job is linked to wider industry trends.
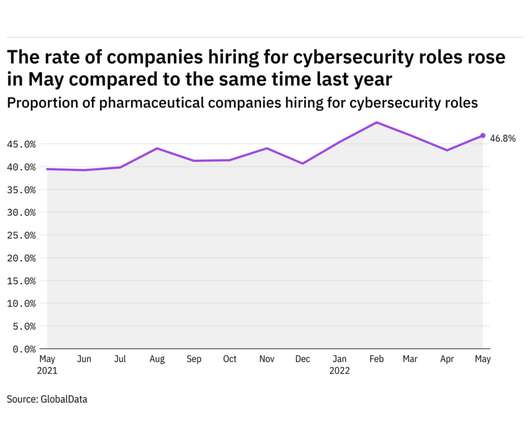
Pharmaceutical Technology
JUNE 27, 2022
GlobalData's job analytics database tracks the daily hiring patterns of thousands of companies across the world, drawing in jobs as they're posted and tagging them with additional layers of data on everything from the seniority of each position to whether a job is linked to wider industry trends. in May 2022.
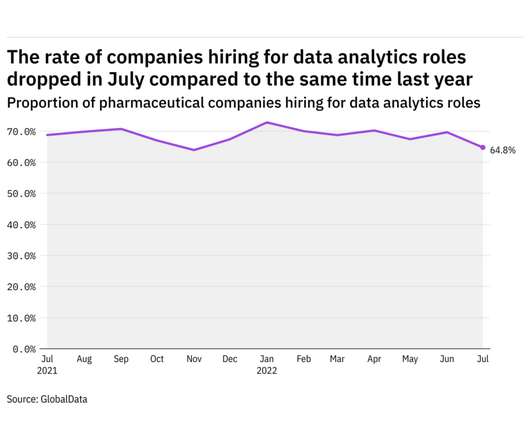
Pharmaceutical Technology
AUGUST 1, 2022
GlobalData's job analytics database tracks the daily hiring patterns of thousands of companies across the world, drawing in jobs as they're posted and tagging them with additional layers of data on everything from the seniority of each position to whether a job is linked to wider industry trends. in July 2022.
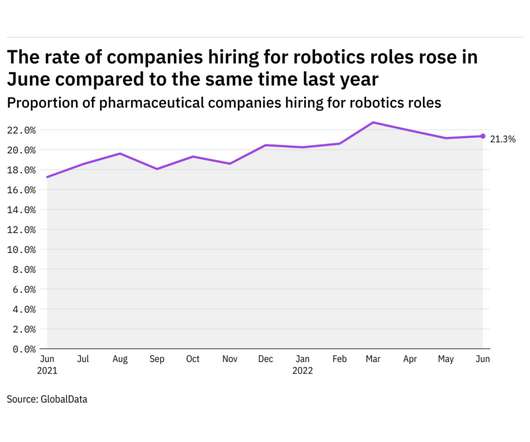
Pharmaceutical Technology
JULY 27, 2022
GlobalData's job analytics database tracks the daily hiring patterns of thousands of companies across the world, drawing in jobs as they're posted and tagging them with additional layers of data on everything from the seniority of each position to whether a job is linked to wider industry trends. in June 2022.
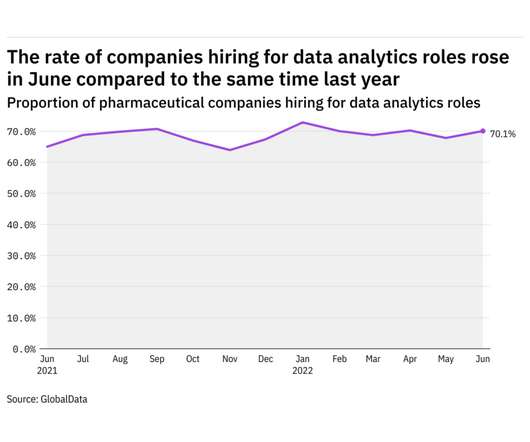
Pharmaceutical Technology
JULY 18, 2022
GlobalData's job analytics database tracks the daily hiring patterns of thousands of companies across the world, drawing in jobs as they're posted and tagging them with additional layers of data on everything from the seniority of each position to whether a job is linked to wider industry trends. in June 2022.
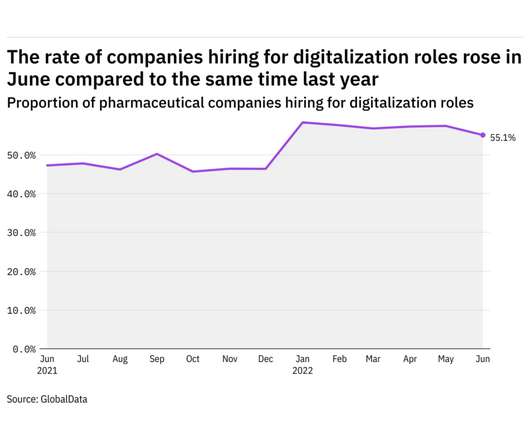
Pharmaceutical Technology
JULY 13, 2022
GlobalData's job analytics database tracks the daily hiring patterns of thousands of companies across the world, drawing in jobs as they're posted and tagging them with additional layers of data on everything from the seniority of each position to whether a job is linked to wider industry trends.
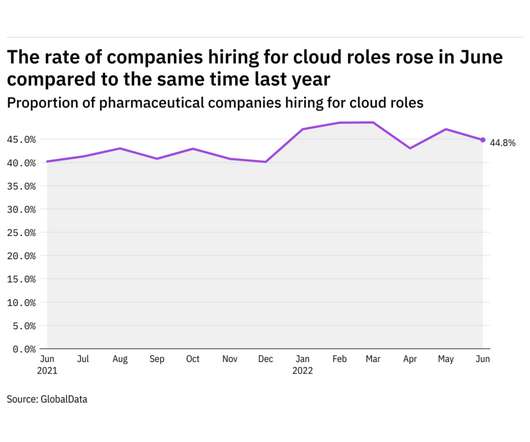
Pharmaceutical Technology
JULY 4, 2022
GlobalData's job analytics database tracks the daily hiring patterns of thousands of companies across the world, drawing in jobs as they're posted and tagging them with additional layers of data on everything from the seniority of each position to whether a job is linked to wider industry trends. in June 2022.
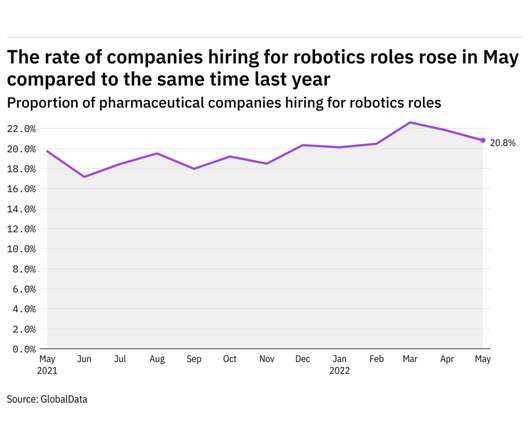
Pharmaceutical Technology
JUNE 23, 2022
GlobalData's job analytics database tracks the daily hiring patterns of thousands of companies across the world, drawing in jobs as they're posted and tagging them with additional layers of data on everything from the seniority of each position to whether a job is linked to wider industry trends. in May 2022.
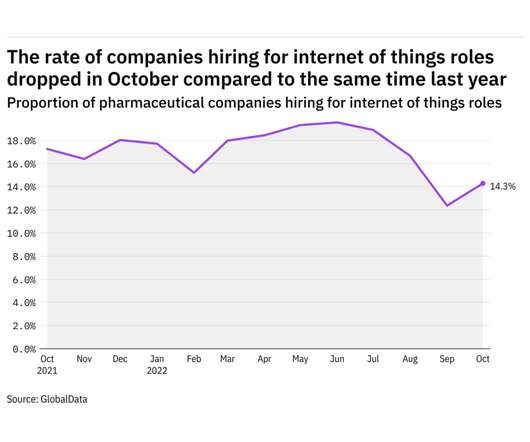
Pharmaceutical Technology
NOVEMBER 8, 2022
GlobalData's job analytics database tracks the daily hiring patterns of thousands of companies across the world, drawing in jobs as they're posted and tagging them with additional layers of data on everything from the seniority of each position to whether a job is linked to wider industry trends. in October 2022.
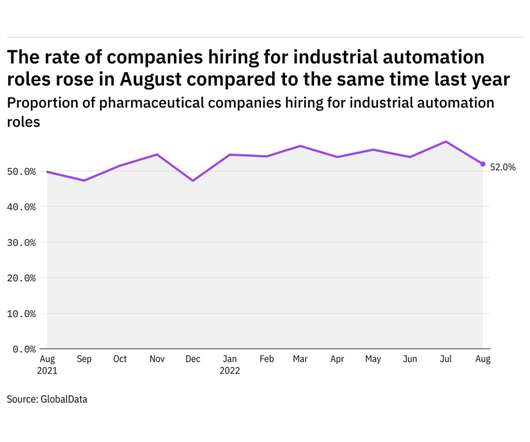
Pharmaceutical Technology
SEPTEMBER 21, 2022
GlobalData's job analytics database tracks the daily hiring patterns of thousands of companies across the world, drawing in jobs as they're posted and tagging them with additional layers of data on everything from the seniority of each position to whether a job is linked to wider industry trends. in August 2022.
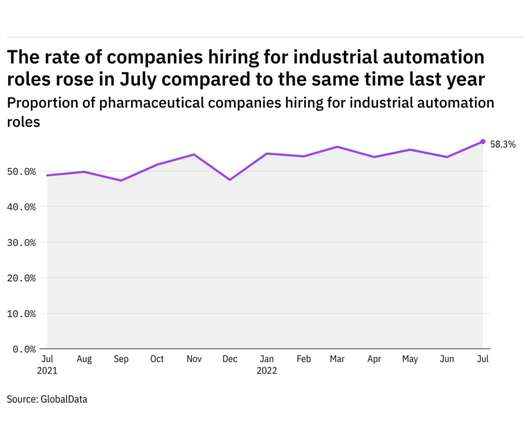
Pharmaceutical Technology
AUGUST 10, 2022
GlobalData's job analytics database tracks the daily hiring patterns of thousands of companies across the world, drawing in jobs as they're posted and tagging them with additional layers of data on everything from the seniority of each position to whether a job is linked to wider industry trends. in July 2022.
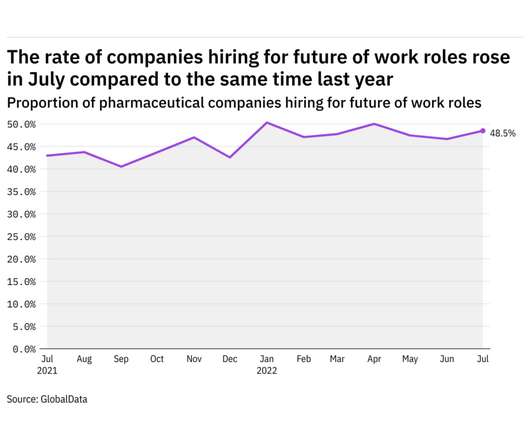
Pharmaceutical Technology
AUGUST 8, 2022
GlobalData's job analytics database tracks the daily hiring patterns of thousands of companies across the world, drawing in jobs as they're posted and tagging them with additional layers of data on everything from the seniority of each position to whether a job is linked to wider industry trends. in July 2022.
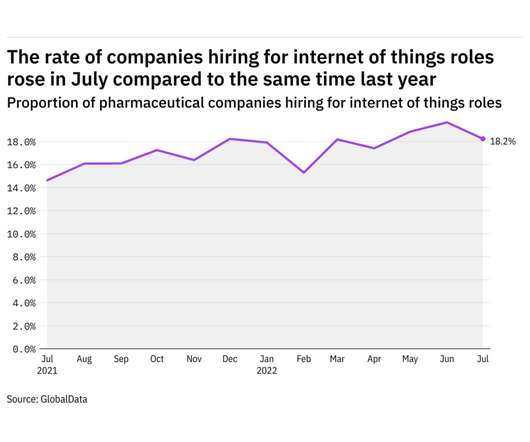
Pharmaceutical Technology
AUGUST 3, 2022
GlobalData's job analytics database tracks the daily hiring patterns of thousands of companies across the world, drawing in jobs as they're posted and tagging them with additional layers of data on everything from the seniority of each position to whether a job is linked to wider industry trends. in July 2022.
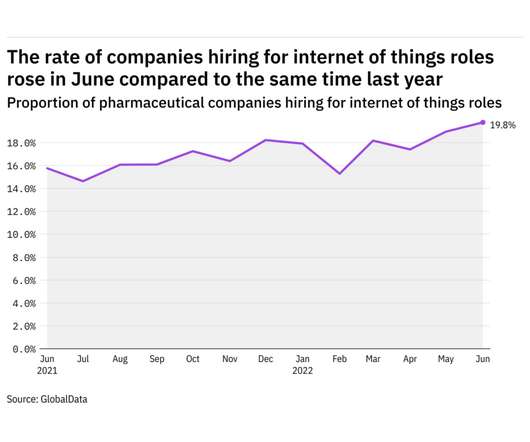
Pharmaceutical Technology
JULY 20, 2022
GlobalData's job analytics database tracks the daily hiring patterns of thousands of companies across the world, drawing in jobs as they're posted and tagging them with additional layers of data on everything from the seniority of each position to whether a job is linked to wider industry trends. in June 2022.
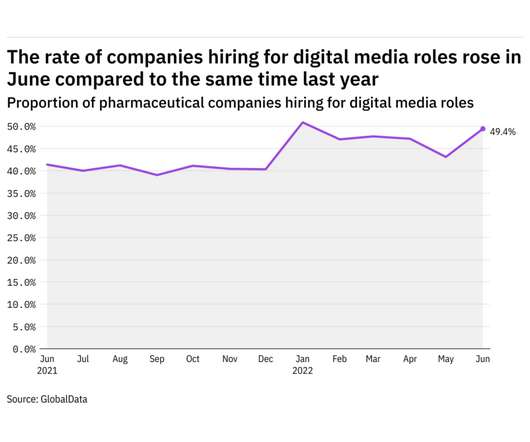
Pharmaceutical Technology
JULY 11, 2022
GlobalData's job analytics database tracks the daily hiring patterns of thousands of companies across the world, drawing in jobs as they're posted and tagging them with additional layers of data on everything from the seniority of each position to whether a job is linked to wider industry trends. in June 2022.

Pharmaceutical Technology
JANUARY 17, 2023
Despite its high price tag, bimagrumab’s unique features set it apart from currently marketed therapies. Mounjaro is anticipated to launch in the US later this year or by early 2024. Bimagrumab is an activin receptor type 2B (ACVR2B or EC 2.7.11.30) antagonist that offers a unique mechanism of action within the obesity market.

RX Note
MAY 1, 2023
However, we should not forget that every luxury or lifestyle choice has a price tag attached to it. Hence, it is worthwhile to keep track of our expenditures and eventually helps us to limit or reduce our monthly expenses. By tracking our expenses, we can easily identify areas where we can cut back to save more money.

Citus Health Specialty Pharmacy
MARCH 3, 2021
Fidelis will also leverage Citus Health for real-time referral, refill production and delivery workflows that allow them to prioritize and track efficiency efforts with customizable forms, App-Less electronic signatures for signed refill and delivery consent and messaging to tag and track key patient data.
pharmaphorum
DECEMBER 23, 2022
price tag of $475,000 when it was first launched in 2017 – and it becomes apparent that these may not be desirable treatment options for every patient and in every setting. Add to this the considerable cost of these medications – the first approved CAR-T, Novartis’ Kymriah (tisagenlecleucel), had a U.S.
pharmaphorum
MAY 10, 2022
The FDA awarded a fast-track designation to lecanemab in December and has also given the drug breakthrough tag as a treatment for early-stage Alzheimer’s, and Biogen and Eisai will be hoping the drug can sidestep the controversy that engulfed Aduhelm from the moment it was approved.
Pharmaceutical Technology
MARCH 22, 2023
The pharmaceutical industry began using radio frequency identification (RFID) tags in the early 2000s. Pfizer was the first to use the tech, adding RFID tags to track a Viagra (sildenafil) shipment circa 2006. Twenty years ago, the cost of implementing RFID tags and the ecosystem (software) was much more expensive than today.
pharmaphorum
JANUARY 31, 2023
What people did not understand was that “fast tracking” approval does not limit the rigorous process involved in ensuring that a medication or vaccine is safe for market use. Given the high price tags placed on many medications, it’s easy to understand why patients often feel that they are being taken advantage of.

epicur
DECEMBER 7, 2023
These tools are tailored to your needs, helping you track stock levels, reorder points, and expiration dates with ease. You can also use shelf labels, reorder tags, or other methods to help set up flags for when hospital supplies and consumables are running low. Haven’t made the switch to digital tools in your practice?

pharmaphorum
NOVEMBER 27, 2022
Fully integrated and automated ingredient to consumer batch tracking using barcoding, RFID tagging, or IoT temperature logging and timestamping significantly reduces the chance of these mix-ups, providing complete traceability records from ingredient manufacturer to patient, and ensures the safety of the product.
Pharmaceutical Technology
MAY 15, 2023
Within the emerging innovation stage, cell therapy for ocular disorders, coronavirus vaccine components, and DNA polymerase compositions are disruptive technologies that are in the early stages of application and should be tracked closely.

Fuld & Company Blog
MARCH 27, 2023
However, there are still plenty of people who are willing to pay out of pocket for semaglutide, despite the high price tag and, hence, could opt to pay for either Wegovy or Ozempic. With trials showing potential weight loss of 10% to 15% , for many, the price tag of $1,500 per month is well worth it.

Pharmaceutical Technology
OCTOBER 10, 2022
It allows business leaders to track the spending on every product feature and simultaneously track the cost of each team. Using sophisticated assignment rules and virtual tagging, each line item inside the unified bill can be assigned to its business role, feature, team, and product.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?

Let's personalize your content