How to conduct product quality review in pharmaceutical
GMPSOP
NOVEMBER 19, 2023
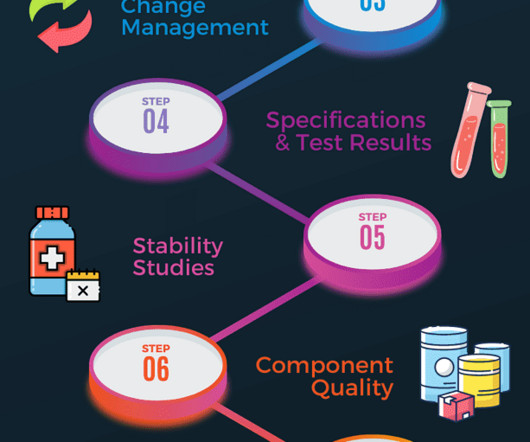
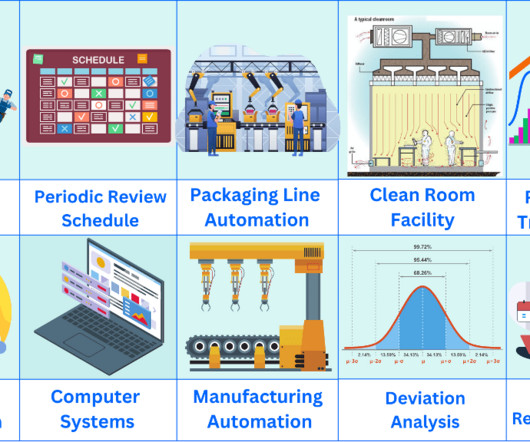
You should perform product quality review annually to determine if any adjustments to product specifications, manufacturing process or raw material supply are required to improve the product further. Product quality review schedule The schedule should be completed on a yearly cycle.








































Let's personalize your content