MD Anderson and Xilis partner to expedite new cancer therapies development
Pharmaceutical Technology
FEBRUARY 16, 2023
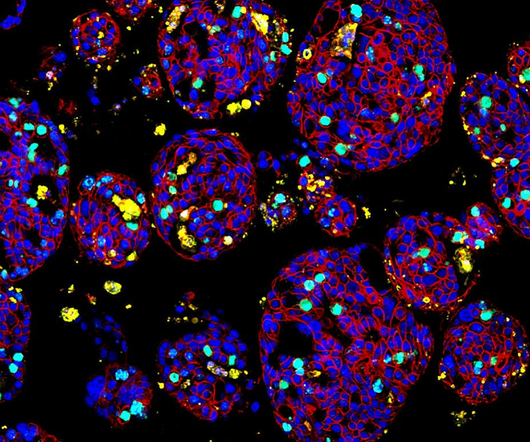
It may offer opportunities for third-party collaborations to guide the new cell therapies and drugs development, if successful. It enables quick evaluation of a patient tumour response to a range of cancer drug modalities within 14 days of obtaining harvested samples of tumour cells.






































Let's personalize your content